What is Frontal Polymerization?
Frontal polymerization is a process in which the polymerization propagates
through the reaction vessel. The three types of frontal polymerizations
are
thermal frontal polymerization (TFP), which uses an external energy source
to initiate the front, photofrontal polymerization in which the localized reaction
is driven by an external UV source, and isothermal frontal polymerization
(IFP), which relies on the Norrish-Trommsdorff, or gel effect, that occurs
when monomer and initiator diffuse into a polymer seed (small piece of polymer).
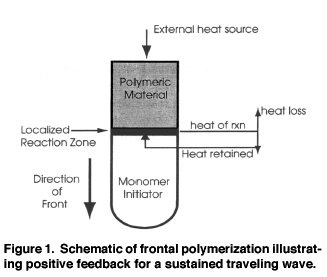
Thermal frontal polymerization begins when a heat source contacts a solution
of monomer and thermal initiator. Alternatively, a UV source can be applied
if a photoinitiator is also present.) The area of contact
(or UV exposure) has a faster polymerization rate, and the energy from the
exothermic polymerization diffuses into the adjacent region, raising the temperature
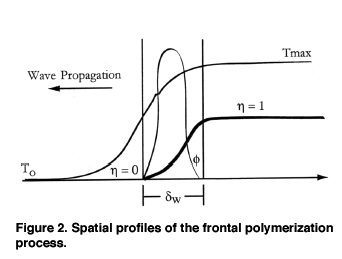
and increasing the reaction rate in that location. The result is a localized
reaction zone that propagates down the reaction vessel as a thermal wave.


(From Washington and Steinbock, 2003)
The simplest example is a front propagating down a glass tube. It is basically a ‘flame’ that propagates through the liquid resin and instead of leaving ash in its wake, the front leaves a solid polymer.
We will refer henceforth to thermal frontal polymerization as just ‘frontal
polymerization’. Frontal polymerization was discovered in Russia by Chechilo
and Enikolopyan in 1972 using methyl methacrylate under high pressure. The researchers
took inspiration from the work by A. Merzhanov and colleagues with Self-propagating
High temperature Synthesis (SHS).
A complete bibliography of all types of of frontal polymerization can be found at this link.
All Movies are in Quicktime. To download the QuickTime plugin.
A 1 cm wide view of a descending front of methacrylic acid. The solid polymer is at 200 ęC while the liquid monomer below is at room temperature. (Pojman, 1991)
A real-time movie of a multifunctional acrylate front initiated by UV light. The system contains a peroxide and a UV initiator. (Nason, 2005)
This is a demonstration of a "deep cure" of a triacrylate. The movies was prepared by a NASA contractor. ISFR stands for In-Situ Fabrication and Repair. The channels between the Plexiglass sheets contain Luperox231 and silica gel (to increase the viscosity).
Cooking Eggs? Sure--using acrylamide & persulfate mixed into roofing tar.